2 4 dnp test|OCR A Level Chemistry Revision Notes 2017 : Tuguegarao DNPH is a reagent in instructional laboratories on qualitative organic analysis. Brady's reagent or Borche's reagent, is prepared by dissolving DNPH in a solution containing methanol and some concentrated sulfuric acid. This solution is used to detect ketones and aldehydes. A positive test is signalled by the formation of a yellow, orange or red precipitate of the dinitrophenylhydrazone. Aromatic c. Explore a curated collection of captivating PMV with Hannah Owo, directly on PMVHaven.
2 4 dnp test,Learn how to use 2,4-dinitrophenylhydrazine (2,4 DNP) to identify and separate aldehydes and ketones. Find out the structure, synthesis, reaction and examples of 2,4 DNP test with Brady's reagent. Learn about 2,4-dinitrophenylhydrazine (2,4 DNP), a compound used to test for ketones and aldehydes. Find out its structure, synthesis, safety and how to do a 2,4 DNP test.Learn how to identify carbonyl compounds using the 2,4-dinitrophenylhydrazine test. Find out the procedure, reagent, precautions and FAQs of this lab experiment.Learn how to test for the carbon-oxygen double bond in aldehydes and ketones using 2,4-dinitrophenylhydrazine (2,4-DNP or 2,4-DNPH). Find out the structure, mechanism and uses of the reaction product, and .DNPH is a reagent in instructional laboratories on qualitative organic analysis. Brady's reagent or Borche's reagent, is prepared by dissolving DNPH in a solution containing methanol and some concentrated sulfuric acid. This solution is used to detect ketones and aldehydes. A positive test is signalled by the formation of a yellow, orange or red precipitate of the dinitrophenylhydrazone. Aromatic c.
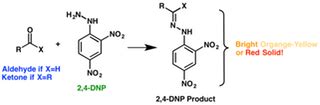
Identify aldehydes and ketones using Brady’s reagent (2,4-dinitrophenylhydrazine) in this microscale experiment. In this practical, students add various liquid aldehydes and ketones to 2,4 .2,4-dinitrophenylhydrazine (also known as 2,4-DNPH) is a reagent which detects the presence of carbonyl compounds (compounds with -C=O group) The carbonyl group of .2 4 dnp test This page looks at the reaction of aldehydes and ketones with 2,4-dinitrophenylhydrazine (Brady's reagent) as a test for the carbon-oxygen double bond. It also looks briefly at some other similar .Shows positive test for: aldehydes and ketones Reactions: reacts with the carbonyl group of aldehydes and ketones How to perform the test: Five drops of the compound to be .Aldehydes and Ketones--2,4-DNP. Hydrazines such as 2,4-dinitrophenylhydrazine react with the carbonyl group of aldehydes and ketones to give colored precipitates. Normally . 2,4 DNP Laboratory Test (Image to be added soon) First, add 5ml of the 2,4-dinitrophenylhydrazine reagent in a test tube. Then, add 10 drops of an unknown compound and sharply tap the test tube with a stick or finger to mix. If the crystals do not form immediately, heat in a water bath (60oC) gently for 5 minutes.
2 4 dnp test OCR A Level Chemistry Revision Notes 2017 The following tests are used to identify the presence of aldehydes and ketones. 2,4-dinitrophenylhydrazine test; Sodium bisulphite test; The difference between ketone and aldehyde is the carbonyl group present .2,4-dinitrophenylhydrazine (also known as 2,4-DNPH) is a reagent which detects the presence of carbonyl compounds (compounds with -C=O group); The carbonyl group of aldehydes and ketones undergoes a condensation reaction with 2,4-dinitrophenylhydrazine . A condensation reaction is a reaction in which two molecules join together and a small .
How to perform the test: Five drops of the compound to be tested are mixed with 5 drops of the dinitrophenylhydrazine reagent (an orange solution) in 2 ml of ethanol and the tube shaken. If no positive test is observed immediately, the mixture should be allowed to stand for 15 minutes. A positive test is indicated by: 2,4-DNP forms an orange ppt with aldehydes and ketone, the mp of which identifies the aldehyde or ketone that was reacted.Private tuition online from frankly. This @TheElkchemist practical video illustrates how you can use Brady's reagent or 2,4-DNPH to test for presence of carbonyl compounds like aldehydes and ket. What is 2,4-DNP Test? The 2,4-dinitrophenylhydrazine (2,4-DNP) test is commonly used in organic chemistry labs to detect the presence of carbonyl groups (aldehydes and ketones) i.e., (C=O) functional group in organic compounds. Basically, the aqueous solution of the 2,4-Dinitrophenylhydrazine is used for the Identification of the .

Ethanol; Propanone; p-Methoxybenzaldehyde (or other aromatic aldehyde or ketone) Methanol; Ethanal (Acetaldehyde) Solution of 2,4-dinitrophenylhydrazine (see preparation notes below) – requires 24 hours to dissolve completely; For preparation of 2,4-dinitrophenylhydrazine

One test is Brady's reagent where 2,4-dinitrophenylhydrazine is dissolved in a solution containing methanol and some concentrated sulfuric acid. 2,4-Dinitrophenylhydrazine or DNPH is a reagent used in organic analysis and detection of ketones and aldehydes.
One test is Brady's reagent where 2,4-dinitrophenylhydrazine is dissolved in a solution containing methanol and some concentrated sulfuric acid. 2,4-Dinitrophenylhydrazine or DNPH is a reagent used in organic analysis and detection of ketones and aldehydes. To take out 2,4-DNP test, five drops of the compound to be tested are mixed with 5 drops of the dinitrophenylhydrazine reagent (an orange solution) in 2 ml of ethanol and the tube is shaken. If no positive test is observed immediately, the mixture should be allowed to stand for 15 minutes for a complete reaction to take out. 2 4 DNP Test: Testing to identify aldehydes or ketones with 2,4- dinitrophenylhydrazine (DNPH) is a convenient way to separate mixture components between aldehydes and ketones. If an aldehyde or ketone .F. 2,4-DNP Test for Aldehydes and Ketones . benzaldehyde, 2-butanone . Into a small test tube, place 20 drops of 2,4-DNP solution. Add 2 drops of the known sample and mix thoroughly by swirling. Observe for the formation of an orange or red-orange precipitate. Record your observations.OCR A Level Chemistry Revision Notes 2017 The 2,4-DNP test, also known as the Brady’s Reagent Test, is like a chemical speed-dating event. It uses 2,4-DNP to identify carbonyl groups in ketones and aldehydes. By forming 2,4-DNP derivatives, chemists can separate mixtures of ketones and aldehydes through column chromatography. After separating the derivatives, hydrolysis . Hello Students, Watch the Complete Video on the 2,4-DNP Test of Aldehydes Ketones Carboxylic Acid Class 12 Chemistry Chapter 12 by Anshu Ma'am. This session . Q) Write a chemical test to distinguish ethanal from ethanol? Ethanal and ethanol can be distinguished by 2,4-DNP test. Ethanal (i.e aldehyde) reacts with 2,4-dinitrophenyl hydrazine (2,4-DNP) to form orange ppt. of ethanal 2,4-dinitrophenyl hydrazone. But ethanol does not give this test. Note: 2,4-DNP = Brady’s reagent.
Test-1: Acetophenone + Ethanol. dissolve + 2,4-DNP gives orange ppt.Test-2: Benzophenone + Ethanol. dissolve + 2,4-DNP gives yellow-orange ppt. This video demonstrates how to test for carbonyls and the expected results using 2,4-dinitrophenylhydrazine
2,4-Dinitrophenylhydrazine (2,4-DNP or 2,4-DNPH) reacts readily with aldehydes and ketones via a condensation reaction (the lone pair of electrons on the terminal amino group in 2,4-DNPH makes it a strong nucleophile and the condensation starts by the nucleophilic 2,4-DNPH attacking the electrophilic carbonyl carbon) to .
2 4 dnp test|OCR A Level Chemistry Revision Notes 2017
PH0 · addition
PH1 · OCR A Level Chemistry Revision Notes 2017
PH2 · Functional Groups
PH3 · Brady’s test for aldehydes and ketones
PH4 · Addition
PH5 · 8: Identification of Unknowns (Experiment)
PH6 · 2,4 DNP Test (2,4
PH7 · 2,4